Back in May of 2009, Johnson and Johnson purchased Los Angeles based Cougar Biotech who has been conducting the clinical trials for the prostate cancer drug for patients with cancer that has progressed to a serious state. Phase 3 of the studies is due to be released in Europe at a cancer meeting in Milan.
Johnson and Johnson Acquiring Cougar Biotechnology – Cancer Biotech Company Los Angeles
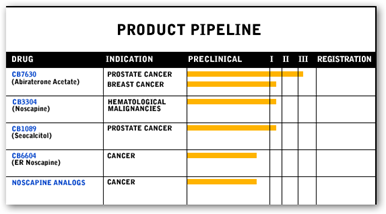
This is good news as many times when trials end so does the patient access so in this case J and J is going to give the participants access to the drug. Below is what Cougar currently has in the pipeline and the drug appears to also be testing for breast cancer. They are also recommending those patients in the trial who received a placebo be given access to the drug as well. An independent committee is recommending the results come forward as participants improvement in overall survival rates and the safety of the drug has been documented. Stay tuned for next month when I’m sure there will be lot more information forthcoming.
In other news J and J has pledged a grant to fund new HIV and TB medication to improve health for up to 120 million women and children in developing nations. One program is called Mobile Health for Mothers and will use cell phones to send text messages to expe4cting moms for coaching. I think this is great for other countries where citizens may have not much more than a phone, but here in the US a multi tasking pregnant Mom might go a little nuts with everything else she may have going on with her phone.
There are other parts to the project that involves distributing drugs to fight intestinal worms and to fight the HIV and TB. Education is included to help women understand how to prevent passing the HIC virus on to their children as well. We get some good news about J and J this week. BD
Ortho Biotech Oncology Research & Development, a unit of Cougar Biotechnology, Inc., today announced that it has unblinded the Phase 3 study of abiraterone acetate plus prednisone for the treatment of patients with metastatic advanced prostate cancer (also referred to as castration-resistant prostate cancer) whose disease has progressed following treatment with one or two chemotherapy regimens at least one of which contained docetaxel.
Study COU-AA-301 included 1,195 patients who were randomized to receive abiraterone acetate plus prednisone or placebo plus prednisone.
The Independent Data Monitoring Committee's (IDMC) recommendation to unblind the study was based on a pre-specified interim analysis, which demonstrated a statistically significant improvement in overall survival and an acceptable safety profile. Based on these results, the IDMC also recommended that patients in the placebo arm be offered treatment with abiraterone acetate.



0 comments :
Post a Comment