Back in April of this year the FDA proposed their increase and now it has been approved and the new costs start next month. You can read the entire listing and how the increases apply here.
The FDA Wants A Price Increase Too – User Fees for Medical Device Companies
Also, earlier this year, the FDA lost one of their chief regulators who went to work at Microsoft and this can actually be good as she will work with Microsoft and help address some of the IT infrastructure at the FDA. BD
FDA Medical-Device Regulator Leaves to Take Position at Microsoft Health Solutions Group – Director of Regulations and Policy
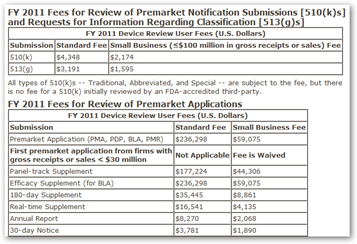
The Food & Drug Administration boosted the user fees medical device makers pay for applications for clearance or approval from the federal watchdogs.
The new fee schedule for fiscal year 2011, which goes into effect Oct. 1, institutes across-the-board hikes of 8.5 percent. Companies must pay an initial fee of $2,179 to register with the FDA.
The agency first announced its plans for the fee hike in April. Medical device companies, whose user fees have funded an increasing share of Food & Drug Administration's device review budget, will have a chance to weigh in on how well the program is working at a Sept. 14 public workshop. The federal watchdog agency will offer its own views and solicit input from consumers, patients, healthcare professionals, scientific and academic experts at the meeting, which is scheduled a full two years ahead of the September 2012 expiration date for the current medical device user fee program.
FDA raises user fees | MassDevice - Medical Device Industry News




0 comments :
Post a Comment