After you check this out, give me yes vote for being able to have bar  codes on products so we can find them easy enough if you would on the poll to the right. Last week it was glucose strips and how long before we get bar codes to help consumers, hospitals and others in healthcare to be able to immediately identify these problems. You can read all the lot numbers here
codes on products so we can find them easy enough if you would on the poll to the right. Last week it was glucose strips and how long before we get bar codes to help consumers, hospitals and others in healthcare to be able to immediately identify these problems. You can read all the lot numbers here
As information here’s the monster recall fro last week. 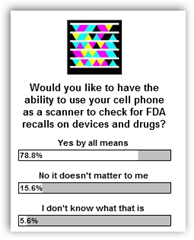 We talk about safety all the time and yet something so doable and a good solution falls on deaf ears. We are not going to fix the recalls anytime soon so let’s not be a BP and let it go until many more die.
We talk about safety all the time and yet something so doable and a good solution falls on deaf ears. We are not going to fix the recalls anytime soon so let’s not be a BP and let it go until many more die.
Abbott Diabetes Care Recalls Tons of Glucose Test Strips–The US Has the FDA Recall Blues-Solution Has Been Touted Here for a Year-It’s Time for a Fix–Readers Have Voted
Can we get some of those luddite pharma companies to get on board, nope. How about those folks that recall devices, have they done anything, nope. That means we now have post # 61 right here for my readers and the deaf ears of others to ignore I guess. Granted this would take a pilot program to begin with but you have to start somewhere. Microsoft Tags can be combined with RFID systems too, which some pharma companies are using, but it doesn’t do anything about recall information and does help recover stolen products. BD
Microsoft Tags on CBS Early Show – Wake Up FDA, Pharma and Medical Device Companies –Scan Those Drugs, Medical Devices and Synchronize with an FDA Tag Data Base – Recalls, Theft Tracking and More….
Audience: Risk Managers, Pharmacists
Issue: American Regent and FDA notified healthcare professionals of the nationwide recall of specific lots of Dexamethasone Sodium Phosphate Injection, USP 4 mg/mL, 30 mL Multiple Dose Vial because some vials of these lots either contain particulates or have the potential to form particulates prior to their respective expiration dates.
Background: This voluntary recall was initiated December 20, 2010 because some vials of these lots either contain particulates or have the potential to form particulates prior to their respective expiration dates. American Regent is undertaking this recall in consideration of the potential for safety issues if these lots of product are administered to patients.Recommendation: The recalled lots are listed in the firm's press release below. Hospitals, infusion centers, clinics and other healthcare facilities should not use the drug product with these lot numbers for patient care and should immediately quarantine any product for return. As stated in the Dexamethasone Sodium Phosphate Injection, USP Product Package Insert, “Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.”




0 comments :
Post a Comment