When you look at the website though instead of the recall having center stage first I  see the notice about the “biggest healthcare spinoff in US history” listed and when you go further down you do see the recall notice on the page. When it comes to news, spinoff trumps recalls and potential dangers it looks like.
see the notice about the “biggest healthcare spinoff in US history” listed and when you go further down you do see the recall notice on the page. When it comes to news, spinoff trumps recalls and potential dangers it looks like.
Recalls of both medical devices and drugs are growing for a number of reasons. First of all, we have a lot more information available today than what we have ever had and we need to capitalize on this opportunity quickly. We read in the news every day it seems about quality control issues, devices needing software updates and ![]() so on. How do we get the word out quickly and efficiently? If one has times they can certainly search the web and put out a full on effort to find all of this every day, but healthcare workers have the same problems we all have and that is time. When human lives are involved, time is everything.
so on. How do we get the word out quickly and efficiently? If one has times they can certainly search the web and put out a full on effort to find all of this every day, but healthcare workers have the same problems we all have and that is time. When human lives are involved, time is everything.
I started this campaign a year ago with lots of support from readers and have sent this to the FDA, DEA and a few drug and device companies. 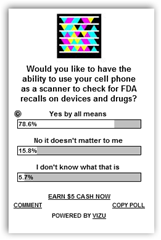 I said a year ago that recalls would accelerate and that we did not need to be the BP of recalls, which that is where are certainly headed. No drug or device company is above this occurring today and many suggest RFID solutions which are good too, but the bar codes go to the web and can offer more detailed information so combine the solutions. It's about time someone does a pilot on this! Everyone who reads here, keep voting!! There are stories and I have posted a couple here in the past where people had died getting implanted with a device that has been recalled and was missed being pulled from inventory and the surgeons had no idea it had been recalled. In the day where we have a technology solution for this, it’s shame luddites still exist and don’t collaborate and pursue answers and prefer to put
I said a year ago that recalls would accelerate and that we did not need to be the BP of recalls, which that is where are certainly headed. No drug or device company is above this occurring today and many suggest RFID solutions which are good too, but the bar codes go to the web and can offer more detailed information so combine the solutions. It's about time someone does a pilot on this! Everyone who reads here, keep voting!! There are stories and I have posted a couple here in the past where people had died getting implanted with a device that has been recalled and was missed being pulled from inventory and the surgeons had no idea it had been recalled. In the day where we have a technology solution for this, it’s shame luddites still exist and don’t collaborate and pursue answers and prefer to put  patients in danger.
patients in danger.
Microsoft Tags – Microsoft MSDN Posts Ideas from the Medical Quack About Use in Healthcare!
We sit around and worry about advertisements and menthol cigarette regulations for example, but we can’t get our nose out to create some real safety solutions. Scan that knee, hip, defibrillator before you use it, takes a few seconds and will help hospital registries function and less mistakes

On another recall from Abbott on glucose testing strips in the news last week look at this massive amount of products that jeopardize consumer safety. Everyone talks but no action from the luddite birds nest today.
Abbott Diabetes Care Recalls Tons of Glucose Test Strips–The US Has the FDA Recall Blues-Solution Has Been Touted Here for a Year-It’s Time for a Fix–Readers Have Voted
Collaboration is just buzz word that everyone uses for their marketing today but nobody does squat to pursue safety technology. I think this is post # 62 that I have made in a little over a year’s time.
Here’s the actual page from the FDA at this link where you can see the multiple lot numbers that are being recalled, there’s a ton of them. Sure would be easier to scan that catheter with a bar code on the packaging. So far though this idea has not had any impact on the FDA and/or drug and device companies and gee they would stand to save money on this process to and of course I have even advocated this for over the counter products too so everyone would win. FDA would have a synchronized cloud data base to check for compliance. Seems like we just prefer to subject people to dangers and not get the information out there and if you look at my poll, consumer want it as well as pharmacists, doctors and hospitals. You know this technology can even be used to authenticate physicians for e-prescribing too, duh!
RAZCODE (Microsoft Tags) Using Smart Phones to authenticate MDs When e-Prescribing Controlled Substances
Also, why not get a heat map and find out where those catheters are, it can be done once the luddites open their ears. Bing maps will do it for you and he’s a screenshot of those who have scanned my bar codes from this blog!
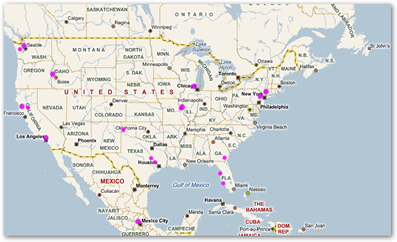
Microsoft Tag Bar Codes–Who’s Been Scanning the Medical Quack–The Bing Heat Map Tells All And Could Help Find Stolen or Expired Drugs and Devices With This Methodology
In the meantime, luddites continue on and the non participants just shine this technology on and we wonder why consumers don’t participate in health technology. It's all about a buck and by the way, watch 60 minutes this week and see the story they have coming up. Here’s the preview below and when recall information is needed, it needs immediate information to be available. The preview here shows a story about the wrong drugs being put in the wrong bottles and yes I commented over there too, doing my best.
If you are a patient undergoing an interventional heart procedure, pray that the defective devices have been removed from the hospital inventory and again I invite you to look at the huge list at the FDA recall press release. Also just from 2 days ago, hope that this drug was pulled from hospital inventories too.
American Regent Recalls Dexamethasone Sodium Phosphate Injections Due to Particulates in Product-Need Barcodes
Would it not be so much easier for someone to take a cell phone and scan their inventory? It only takes a few minutes for updated information  to be sent to the bar codes as again they can be updated at any time.
to be sent to the bar codes as again they can be updated at any time.
I can go to Best Buy and use this technology to find out about a Canon Printer though! We live in foolish world of luddites at times. BD
FOR IMMEDIATE RELEASE – Plymouth, MN – December 15, 2010 - ev3, a Covidien company, has initiated a voluntary recall of specific lots
of the NanoCross™ .014" OTW PTA Dilatation Catheter due to the potential for the catheter shaft to crack or break during use. Cracking or breaking of the catheter shaft may result in the inability to inflate or deflate the balloon, and may result in material separation and potential embolization. The device failure may lead to unplanned intravascular or open surgery, significant vasospasm, prolonged tissue ischemia, tissue, injury, infarct, bleeding and/or death. The Food and Drug Administration (FDA) has classified the recall as a Class I recall. FDA classifies a recall as Class I when the agency believes there is a reasonable probability that the use of the recalled product will cause serious adverse health consequences or death.
The NanoCross 0.014" OTW PTA Dilatation Catheter is intended to dilate stenosis in the iliac, femoral, ilio-femoral, popliteal, infra-popliteal, and renal arteries, and for the treatment of obstructive lesions of native or synthetic arteriovenous dialysis fistulae. The NanoCross Dilatation Catheter is not an implant. It is removed and discarded after the procedure is completed.
ev3 Initiates Voluntary Recall of Specific Lots of NanoCross™ .014" OTW PTA Dilatation Catheter



0 comments :
Post a Comment