I think they should have their day in court to submit their findings and substantiate  the use of Avastin for breast cancer. This is not a new product and has been used to treat breast cancer and it has helped many women. Now what other data could the FDA have looking at? Did one of the biggest health insurers lobbyist get in there with their report on how doctors were not following directions for the treatment of breast and colon cancer? It does make you wonder? This is the first time such a hearing has been requested by a pharmaceutical company with the FDA.
the use of Avastin for breast cancer. This is not a new product and has been used to treat breast cancer and it has helped many women. Now what other data could the FDA have looking at? Did one of the biggest health insurers lobbyist get in there with their report on how doctors were not following directions for the treatment of breast and colon cancer? It does make you wonder? This is the first time such a hearing has been requested by a pharmaceutical company with the FDA.
This was all over national news and every oncologist got a copy of United telling oncologists they were not doing their job right. I had one doctor write to me and he didn’t think that the number of patients cited actually were given approval to receive Avastin and questioned their study. United was right there with questions on the use of Avastin, so again where did this information come from?
United HealthCare Sends Oncology Reports to Doctors – Assessing Cancer Treatment Rules Compiled by Ingenix
We also know they don’t like women to have the gene test for breast cancer either as it is considered too expensive in their book and granted the drug companies could think about the possibility of reducing the cost of the drug as well.
The Genomic Test for BRACAnalysis (Breast Cancer) To Be Scrutinized by United Health Care
Some critics say that monitoring quality is not the appropriate role for an insurer, which has a financial interest in all this. "This is one area I'd rather have doctors police themselves than have an insurance company do it," says Eric Winer, chief scientific adviser for Susan G. Komen for the Cure, a research and educational organization. You can see by the chart below how the doctors were graded and next they will begin looking at Avastin for lung cancer, just wait.
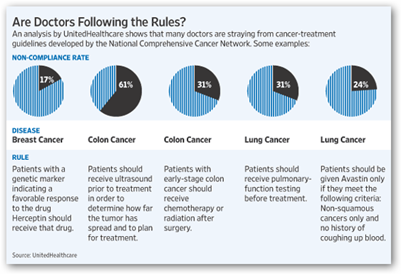
Genentech should at least be able to dispute and see what numbers the FDA has and how their study results came about. I’m not just too trusting of an insurance company who bought up a company in China that already has a line of communication with the FDA to promote more Chinese Drugs and Devices in the US. Ingenix as we hear a lot about today are the data division of United and are mingled in everywhere with stats and numbers even to have been said to help provide the decision making analysis for Arizona to cut off transplants for those with hepatitis C. They don’t attach names to treatments, just there to save money.
UnitedHealth subsidiary (Ingenix Subsidiary I3) Acquires ChinaGate – Working to Sell Chinese Products Globally
This furthermore makes the point of why drug companies should be able to negotiate directly with Medicare too. Granted the drug is high priced but when all sit down and look at reality with real studies, and not those algorithms that produce “desired” results just to save money, we should be able to get some reality on the table here. Again, just my feeling here that some of these numbers may have crept over to the FDA and they may have addition information, but I would like to know if this got in the pot out of curiosity. This pilot program of putting oncologists on pay for performance gets my suspicions going where it is lead by United and Aetna is participating too with rewarding oncologists for saving money in oncology, and this is a touchy area all the way around and if not done correctly, these are  death panels.
death panels.
Health Insurers Focusing on Cancer Treatments - Pilot Programs To Follow Standard Treatments & New Payment Structures
United also has pharmacists on pay for performance too at Walgreens to sign up individuals in their various programs. BD
Genentech Inc. filed a formal request Thursday with the Food and Drug Administration for a hearing on the agency's plan to rescind approval of the world’s best-selling cancer drug, Avastin, for women with metastatic breast cancer.
In a letter from Michelle Rohrer, the South San Francisco-based biotech company’s vice president of regulatory affairs, Genentech asks FDA Director Janet Woodcock for a hearing on the use of Avastin with the chemotherapy agent paclitaxel. The company said it will submit supporting documentation by Jan. 18.
Genentech asks for Avastin hearing | San Francisco Business Times



0 comments :
Post a Comment