Last time I wrote about the IPhone Heart Monitor the company while waiting for the FDA released a version for cats and dogs and what a great idea. It provides clinical quality data. The cost of the product is $199 and will require medical identification to show one is a doctor, 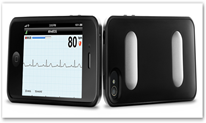 in other words this is not intended as a consumer product yet. The next 501k approval is needed from the FDA to make that move. It detects arrhythmias and in time prescriptions for the device can be made by doctors for those who have atrial fibrillation and are under medical treatment plans. Again they are in pursuit of having an over the counter device available sometime next year. The device snaps onto an IPhone like a case and there’s no wireless pairing required.
in other words this is not intended as a consumer product yet. The next 501k approval is needed from the FDA to make that move. It detects arrhythmias and in time prescriptions for the device can be made by doctors for those who have atrial fibrillation and are under medical treatment plans. Again they are in pursuit of having an over the counter device available sometime next year. The device snaps onto an IPhone like a case and there’s no wireless pairing required.
Here’s a raw video showing the use with a dog and a horse. BD
While Waiting for FDA Approval for an IPhone ECG Company Launches Popular Veterinary Version–Cats and Dogs Get Heart Attacks Diagnosed

The FDA has granted a 510(K) Class II clearance to San Francisco-based AliveCor’s iPhone-enabled heart monitor, which has been commonly known as the “iPhoneECG” since it first made an appearance at CES two years ago. The company announced the clearance as the mHealth Summit kicks off this week in the Washington DC area. AliveCor will begin pre-selling the $199 clinical-quality, ECG monitor, which has the form factor of an iPhone case that fits iPhone 4 and 4S devices, directly from its website starting today, December 3rd.
http://mobihealthnews.com/19306/fda-clears-alivecor-heart-monitor-doctors-can-pre-order/




0 comments :
Post a Comment