This product is not FDA approved for use in the US yet, but it appears it could be getting closer. Anything that could help 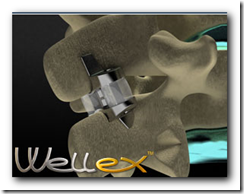 avoid fusion surgery is certainly something that can and will benefit all. The company markets fusion products now and this looks to be a move into the non-fusion area which will enable patients to have additional mobility after a procedure. BD
avoid fusion surgery is certainly something that can and will benefit all. The company markets fusion products now and this looks to be a move into the non-fusion area which will enable patients to have additional mobility after a procedure. BD
Eden Spine announced today that it has received CE Mark approval for its proprietary interspinous medical device, the Wellex". Developed in collaboration with Dr. Jean-Marc Fuentes from France, the Wellex is based on two decades of clinical experience with nonfusion devices. "The Wellex is a compressible dynamic extension controller that not only relieves pain but also positively affects the long-term health of the segment," said Dr. Fuentes, "through its ability to dynamically control extension while protecting and maintaining the neutral zone.
Eden Spine has developed three new-generation, nonfusion technologies - the WellDisc total disc replacement; the FX 1 Dynamic Rod; and, the Wellex Interspisous Technology. As a Sales & Marketing organization, Eden Spine distributes in the U.S. a comprehensive range of FDA-cleared fusion technologies.
Eden Spine Receives CE Mark For Its Nonfusion Wellex" Interspinous Process Medical Device



0 comments :
Post a Comment