This is a whole new open territory for the FDA and as I have mentioned before as late as 2008, all the investigators and analysts didn’t have computers, but all that aside, how is wireless going to be approved by the FDA. This is a tricky one to say the least, not tricky per se in what it does  but they also have to make sure the data is going in the right direction.
but they also have to make sure the data is going in the right direction.
Below I have included some related information from another article, which speaks about a trial with Novartis, a pharma company and their 20 person trial with an implant with each patient for taking blood pressure medication. What pharma and insurance companies want is “compliance” to continue to yield profit, and if it helps the patient along the line, then it appears to be a win-win. Both entities have enjoyed very large profits over the years and the weaning process of getting less for both is tough.
The trial used the “Pill on a Chip” process, where each pill contained a chip, and the patient had a chip inserted under their skin, so when a patient took the pill with the chip, it talked to the chip inserted under their skin and sent the “DATA” back through a cell phone to those collecting the  information. You can read more about the VeriChip link below that connects to PHRs.
information. You can read more about the VeriChip link below that connects to PHRs.
VeriChip Implant Links To Your PHR Health Records, Credit History, Social Security With PositiveID
Also mentioned is Healthphone, who’s site was down at the time of this writing, but has a long time relationship with Microsoft according to this article.
This week I had also posted about another compliance article, a pill bottle that talks to your cell phone. I will make one statement here with all of this connectivity, get a PHR and do not use a device unless it uses the PHR Gateway. Why, so you can remain in charge and cut it off when you want. Of course this would not necessarily be the case with items such as an implanted defibrillator, but those devices that measure compliance and the ones that are not life and death, go the PHR route to protect your privacy. The link below gives the details on how your information also gets used by the pharmacy and helps you along to having your medication history sold. Do you care, well you should as you really need to know who has it. Remember your compliance and medications can go right back in detailed data report to the insurance companies. 
The Pill Bottle That Talks To Your Cell Phone, Creates Data Reports and More…
All of this technology with a not so smart Congress bothers me a bit having written code as I have a pretty good ability to project where the next steps are with integration since I did that for a while. Insurance companies want their data and devices that report data are the ultimate data orgasms for them if you will, as they can pin and nail the numbers down to the exact 10th of a second for all your activity. When there are questions and conflicts they mine through it and chances are pretty good you will not come out a winner with debates.
No wonder the FDA is in a quandary over some of this, are they responsible now for privacy and grant the approval for use of the wireless devices when it ends up selling your information? Give that one some real thought. These are basically individuals at an agency that have been responsible for chemical related products for years, drugs, and some medical devices, so their area of expertise has had to expand as well.
The theme of Proteus is Intelligent Medicine and you can watch the video here. We talk about integration with software on our computers, well their goals are to integrate devices, as you can see in the couple screenshots, will your pacemaker also control your diabetes? Will your hip implant be talking to a monitor you stick in your shoe? There’s no limit on some of this stuff and yes it will get crazier and then this moves into real life, that’s when we need to worry and read up to be prepared.
These devices all send text messages with the intention of making you a better patient and reminding you, but let’s say you have an iPhone that you use to check in with wellness coaches 4 times a day wirelessly to let wellness coaches know your activity level to give you discounts on your health insurance, and then you have an implant with texting pills, and maybe even a defibrillator that talks to the iPhone, and then you need to remember to take your glucose each day so the phone and device text you as well, so now you have an army of devices bugging you constantly. If you were working in a retail store, forget helping the customer when they need it, you have to take care of your health and be on time as the compliance managers are watching! Gee!
Back on track with the FDA, if they approve products that cause insanity, well they don’t want that responsibility either for symptoms of such to be caused by those only on the money trail and wanting sales. Again, so many of the companies who create such products all have their own software  and tell you, no sweat, your doctor is going to monitor all your conditions and take real good care of you. Well he/she’s no superhuman, so throw out about 12 different types of data software for him to check in with, or worse, getting all of your messages sent to his phone, he’s overdone and has no time to see patients any longer. If they have an EMR that connects with this data they may have more of a standing chance, but still they are not going to be able to see every piece of data the second it is released. If there is a 3rd party who collects and analyzes the data before going to your doctor, find out who, what and where they are. There’s all kinds of advertising with some of the devices and you need to read the fine print and check out all their advertising or other partners so you know up front what is happening with your data.
and tell you, no sweat, your doctor is going to monitor all your conditions and take real good care of you. Well he/she’s no superhuman, so throw out about 12 different types of data software for him to check in with, or worse, getting all of your messages sent to his phone, he’s overdone and has no time to see patients any longer. If they have an EMR that connects with this data they may have more of a standing chance, but still they are not going to be able to see every piece of data the second it is released. If there is a 3rd party who collects and analyzes the data before going to your doctor, find out who, what and where they are. There’s all kinds of advertising with some of the devices and you need to read the fine print and check out all their advertising or other partners so you know up front what is happening with your data.
Unless the data reporting device situation is coordinated with better implementation methodologies, you may end up someday having to wait at Verizon or AT &T for an “FDA approved” cell phone too. Right now the market is to sell, sell, sell and hey if you can be cornered into insanity with a dozen devices, they’ll do it, so again use common sense and get involved. Demand that your information be routed through a PHR first so you are in control.
FDA Trying to Figure Out Web 2.0 – Public ...
Devices are not a bad thing, but like everything else today they can be over marketed and you could be driven to the point where you may have more than what you need, just think if yourself as “human email” and that will make the point very clear. We are all over loaded with email. So for wireless and the FDA I don’t see the conservative approach moving a lot faster and for good reason, but by the time it may be approved, there may also be a better and improved product out there too, but that’s how it works with research and development in today’s world, drugs too. BD
Computerworld - SAN DIEGO -- Makers of wireless electronic health devices bemoaned the federal product approval process and stingy insurance reimbursements for life-saving technology that they argued could reduce overall national health-care costs.
Despite a federal priority on health care reform, the U.S. Food and Drug Administration is not doing all it should to boost approval of wireless e-health devices for monitoring patients, said Eric Collins, CEO of Montage Systems, during a panel discussion at the International CTIA Wireless I.T. and Entertainment conference.
The wireless e-health industry "has a huge opportunity to improve the health care system and reduce costs, but Washington is clearly not
paying attention to technology at this level, " Collins added during Thursday's panel presentation.
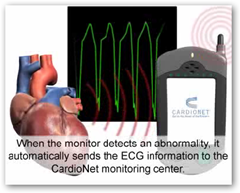
Aaron Goldmuntz, director of business development for CardioNet, said his company began offering wireless cardio monitoring technology nearly a decade ago and described FDA approvals of the technology as having generally gone smoothly. However, his company recently faced a reduction in insurance reimbursements for which CardioNet patients are eligible.
Collins said he is trying to get FDA approval for the Healthphone, a handheld device about the size of a pager that gathers data wirelessly from a patient's body and forwards it over a network to a computer monitored by doctors or other caregivers.
A related function calls for using the device to monitor an orthotic device inserted in a diabetic patient's shoe which would notify the patient wirelessly if a pebble or other foreign object entered the shoe that could cause a sore.
Makers of wireless health devices bemoan slow FDA approval process
Pharmaceutical giant Novartis has tapped intelligent medicine start-up Proteus Biomedical for a small 20 patient study to track patients’ compliance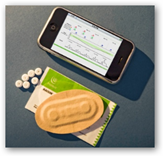 with their blood pressure drug regimen. The patients are taking blood pressure drug Diovan and the study organizers track their compliance via Proteus’ “chip in the pill” technology, which reports to a receiver sensor on the patient’s shoulder when the medication has been ingested. The study has improved compliance from 30 percent to 80 percent after six months, according to Novartis.
with their blood pressure drug regimen. The patients are taking blood pressure drug Diovan and the study organizers track their compliance via Proteus’ “chip in the pill” technology, which reports to a receiver sensor on the patient’s shoulder when the medication has been ingested. The study has improved compliance from 30 percent to 80 percent after six months, according to Novartis.
Proteus’ Raisin technology runs on an electric charge generated by the patient’s stomach acid. The charge is detected through the patient’s body by a sensing patch on the patient’s skin. The patch records the time and date that the pill is digested and also measures some vitals like heart rate, activity and respiratory patterns. The information is then sent to the patient’s mobile phone and then onto the internet for caregivers to review and analyze.
http://mobihealthnews.com/4513/novartis-proteus-pilot-to-lead-to-exclusive-deal/



0 comments :
Post a Comment