If you are not up to date on the Triad recalls, read the post below as this is huge,  affects consumers, hospitals, doctors offices, medical device manufacturers and more. Perhaps my 2 year campaign of almost 2 years now will get some attention and finally do something for consumers.
affects consumers, hospitals, doctors offices, medical device manufacturers and more. Perhaps my 2 year campaign of almost 2 years now will get some attention and finally do something for consumers.
The article was in the Milwaukee Journal Sentinel Paper and first of all I would like to say thanks for the reporter, Rick Barrett for referencing and calling the Medical Quack after finding my articles on the subject of bar codes for recalls. Here’s my contributions and the article is very well done with a full investigative report here with 2 reporters working on the entire article.
“It would take health care workers only a moment to scan something, such as a box of disposable alcohol pads, to determine whether it's on a national recall list, according to Barbara Duck, a California-based medical bar code advocate who  has written medical records software for doctors.
has written medical records software for doctors.
They could even use their smartphones for the task - but only if the recall information were readily available and bar codes were programmed to display it. Now, the technology is used more for inventory control and sales purposes than to send out recall alerts.
"I can take my smartphone to a Best Buy store, scan something like a Canon printer, and it tells me everything about it," Duck said . "But when it comes to a product recall that might save my life, I can't get anything."
She believes that hospitals have missed product recalls when a simple bar code  could have alerted them to the information.
could have alerted them to the information.
"One company found that even after a medical device with a potentially dangerous flaw was pulled from the market, doctors at more than 40 hospitals implanted it in at least 50 patients," Duck said.
Finding information on recall notices on the FDA website can be difficult.
"Users may review hundreds of serial numbers and/or lot numbers published by the FDA and companies," Duck said. "Then they have to manually compare the information on a product label."
This was so bad with the bacterial contamination of the products that the FDA had to shut the place down and then we found out all the big companies they private label for as shortages starting appearing everywhere from CVS and Walgreen’s shelves to hospitals.
FDA Moves in on Triad  and Seizes $6 Million Dollars of Product-The Medicated Wipe Recalls Issues Continue - Triad is an Outsource for Several Fortune 25 Companies
and Seizes $6 Million Dollars of Product-The Medicated Wipe Recalls Issues Continue - Triad is an Outsource for Several Fortune 25 Companies
Here’s a little more history on the recall efforts and again the FDA does nothing to include even giving me an answer. It takes them 2 weeks to send a “canned” response when most websites do that immediately.
FDA Announces Recall of Alcohol Prep Pads, Swabs, Swabsticks From Triad–FDA and Manufacturers Should Ashamed-Campaign for Bar Codes Still Stands Stronger Than 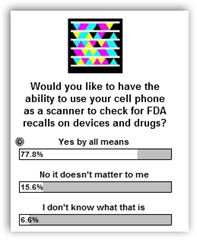 Ever!
Ever!
This is just a shame as nobody wants to make it easier for the consumer and the problem is “non participants executives and lawmakers” who hold jobs that over see this and it certainly shows a lack of digital literacy or desire to help consumers. I even gave the FDA the big luddite recognition in this area that they seem to draw. I have even done a a survey here and still nothing from the FDA. Consumers, pharmacists, doctors, hospitals, Health IT and more were all high on the idea and I tons of tweets on Twitter.
Recalls: FDA Gets the Big Luddite Award with Drug and Device Companies That Do Little or Nothing to Help Consumers-Triad Products Could Have Been Bar Coded To Scan
I just kind of wonder if J and J got spooked with their Baby Wipe products and tried  to give this a whirl, just in case they had to recall anything as they are right in here too. Right now the bar codes contain product information but they could be reprogrammed to give real time recall information if that were to occur. Yes I have beat J and J over the head too with this idea in more ways than one.
to give this a whirl, just in case they had to recall anything as they are right in here too. Right now the bar codes contain product information but they could be reprogrammed to give real time recall information if that were to occur. Yes I have beat J and J over the head too with this idea in more ways than one.
Johnson and Johnson Puts Microsoft Tag Bar Codes on Baby Wipes But Can’t Do the Same to Give Consumers the Chance to Find Their FDA Recalls - BarCode Baby Steps?
If all of this is not compelling enough, read my article below on how a man died being implanted with a device that mal functioned, an error that could have been avoided. Many hospitals have RFID system which work pretty good with finding recalls, but that is not most and it does nothing for the consumer, pharmacist, doctor or small outpatient surgery centers. We all are affected. We just have flaming luddites around and those who are only money oriented who can’t see the forest for the trees and see the return on investment cost has here, luddites and “non participants” with consumer Health IT technology.
There are two links at the top of the site where you can read up and find out more. I think I have about 75 articles or so, every time there’s a substantial recall where the technology would have helped, I have been on it. Again, be sure to read the  entire article here as they did a real nice job summarizing additional areas beyond what I cover here as well with a lot of history. By the way, India has spoken out too about beginning to use the 2D bar codes on exports soon too. Even UPS has given the FDA some good idea on using bar codes as logistics companies are right on top of this and have used the technology almost longer than any other. I guess the FDA as well as drug and device companies look at dollars first before safety. You can scan the dotted bar code at the right and read the Medical Quack on your cell phone at any time:) BD
entire article here as they did a real nice job summarizing additional areas beyond what I cover here as well with a lot of history. By the way, India has spoken out too about beginning to use the 2D bar codes on exports soon too. Even UPS has given the FDA some good idea on using bar codes as logistics companies are right on top of this and have used the technology almost longer than any other. I guess the FDA as well as drug and device companies look at dollars first before safety. You can scan the dotted bar code at the right and read the Medical Quack on your cell phone at any time:) BD
More than six months after a worldwide recall, potentially deadly alcohol wipes remain in personal medicine cabinets and possibly on store shelves.
The U.S. Food and Drug Administration, tasked with protecting public health, says the recall was effective but refuses to release audit reports detailing findings and measures taken by the company that made and distributed the wipes: Hartland-based Triad Group and its
manufacturing arm, H&P Industries.
Meanwhile, another company that hospitals and others have turned to for supplying wipes since the recall has its own history of manufacturing problems, twice in the last 18 months recalling wipes that didn't meet specifications, the Journal Sentinel has learned.
Further complicating matters is a disjointed product coding system that hampers the ability of hospitals, distributors and the public to identify and track recalled products.
"It's alarming," said Mary Ann Beaumont, a Milwaukee resident who had a package of recalled swabs at home and only learned of the contamination months after the recall when she read a story in the Journal Sentinel in June.



 Ever!
Ever!

0 comments :
Post a Comment