For all of those who have undergone the injections, it appears to be getting closer to a time when the medication would be applied as a cream by a physician. Revance Therapeutics has posted some results of phase 2 of the trials.
Compared to an injection, if the process is good enough who would choose an injection versus a cream, although there’s no word on how many applications would need to be applied, but still it sounds good. I also recently read where women are also getting botox injections in their feet from wearing high heels too, but I would think the foot area would require some monitoring and advice from a physician too as you would not want to perhaps over look other foot problems that could be arising from diabetes as an example and perhaps accidentally mask any symptoms there. BD
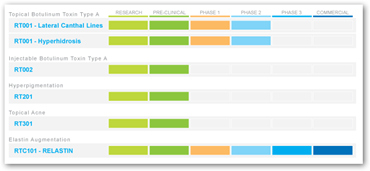
The first application of the TransMTS™ technology is a topical botulinum toxin type A for the treatment of moderate to severe lateral canthal lines (crow's feet). RT001 is an investigational product in clinical stage development under an IND filed with the US FDA.
RT001 demonstrated statistically significant efficacy versus placebo and achieved the primary and multiple secondary endpoints in the most recent US Phase 2b study. It appears to be safe and was well tolerated in over 200 patients in studies in the US and internationally




0 comments :
Post a Comment