Anyone who reads the blog regularly knows about my 6 month plus campaign where I have been advocating 3D and2D barcodes, also known as Microsoft Tags. If you 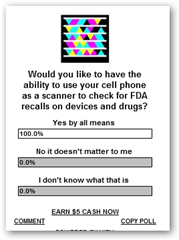 have a chance enter your vote before you leave this page and let me know if you would value being able to use your cell phone as a scanner to find recalled products, drugs, devices etc. right on the shelves.
have a chance enter your vote before you leave this page and let me know if you would value being able to use your cell phone as a scanner to find recalled products, drugs, devices etc. right on the shelves.
Microsoft Tags in Healthcare – Comments and Demonstration at “Microsoft Connected Conference” with Surface
Now with this data base they are creating, why not put a Tag on each page and have the device and drug companies synchronize their updates with the FDA? This also gives the FDA an answer to monitoring compliance with checking to see if the “tags” have been updated, what a novel idea use some technology here, right? This is not much different than faxes were in the early days as we had people skeptical about those too and now look where we are.
This is nice that websites can use the downloadable XML format, but what the heck am I going to do with an XML formatted download while I’m shopping? Duh?
Microsoft Tags – Microsoft MSDN Posts Ideas from the Medical Quack About Use in Healthcare!
Recalls of both medical devices and drugs are growing for a number of reasons. First of all, we have a lot more information available today than what we have ever had and we need to capitalize on this opportunity quickly. We read in the news every day it seems about quality control issues, devices needing software updates and so on. How do we get the word out quickly and efficiently? If one has times they can certainly search the web and put out a full on effort to find all of this every day, but healthcare workers have the same problems we all have and that is time. When human lives are involved, time is everything. I wrote my original post at the link below back in October of 2009 so you can see how slow we move.
Tracking Medical Device Recalls – Sounds Like A Good Place for a Microsoft Tag Data Base at the FDA
Scan that knee, hip, defibrillator before you use it, takes a few seconds and will help hospital registries function and less mistakes. Hospitals work hard to do a good job at this, but I continue to read stories to where patients have been implanted with a device that had been recalled and it was missed. One story in particular involved a man who was implanted with a heart device that had been recalled and he died when it malfunctioned. To me, this could have been a preventable incident and a life could have been saved if a simple scan would have put up the red flags to not use the device. You can find the full summary article at any time on the blog as it has a permanent resting spot on the title section, just look for this bar at the top of the page.
 Would it not be nice at the FDA to get an automated alert every time a company does an update instead of having to chase it down with either phone calls or long extensive web searched? Of course it would and we get to kill many birds with one stone here as consumers benefit, FDA benefits and so to the manufacturers in getting the word out on recalled products. Also, it would help the drug enforcement agencies find counterfeit drugs/devices, no tag that matches spec and takes you to the appropriate page, then watch out as each tag could contain it’s own FDA token to protect against those trying to create fake Tags, as that will occur.
Would it not be nice at the FDA to get an automated alert every time a company does an update instead of having to chase it down with either phone calls or long extensive web searched? Of course it would and we get to kill many birds with one stone here as consumers benefit, FDA benefits and so to the manufacturers in getting the word out on recalled products. Also, it would help the drug enforcement agencies find counterfeit drugs/devices, no tag that matches spec and takes you to the appropriate page, then watch out as each tag could contain it’s own FDA token to protect against those trying to create fake Tags, as that will occur.
The Tags will be the way to easily scan and enter information into your PHR too, so think about a simple technology that has a lot of answers. We are all not going to live our lives on internet doing extensive searches as the world doesn’t work that way today, we want it now and this technology can do it. I certainly have some biotech companies reading up here on their use too and there’s work in progress to use the Tags to authenticate e-prescribing, so again what more do you want with many answers with one simple technology. BD
RAZCODE (Microsoft Tags) Using Smart Phones to authenticate MDs When e-Prescribing Controlled Substances
Please remember to vote! Thanks much!
The Food & Drug Administration plans to launch a public database of recall data this fall, another element in the agency's quest to become more transparent.
The data, on recalls of medical devices, food products and drugs, will be compiled in a searchable, online database. The data will also be made available in a downloadable XML format.
The information to be posted will include brand and company names, product descriptions, the problem that prompted the recall and product photos when available, FDA transparency coordinator Afia Asamoah told the Sunlight Foundation.
FDA plans public recalls database | MassDevice - Medical Device Industry News





0 comments :
Post a Comment