The FDA here in the US has not cleared the entire Proteus system for use with the  chip but did give approval to the wireless monitoring device portion; but the folks in Europe get to have the first crack at taking a pill that will send back an audit trail of all the information.
chip but did give approval to the wireless monitoring device portion; but the folks in Europe get to have the first crack at taking a pill that will send back an audit trail of all the information.
FDA Clearance of Wireless Proteus Wireless Monitoring Device Reported – Novartis and Medtronic Have $50 Million Invested in the “Raisin”
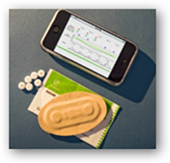
The pill can talk to your blue tooth phone or any other blue tooth device. Novartis has invested a few dollars with the technology. Novartis is continuing with investing with Proteus to have a chip on pill that will  report data. The technology is called “ChipSkin”. How does this work, you swallow your pill and your pill starts talking and sending information. The information from the pill goes to a patch or a tiny device implanted under your skin and will send your heart and respiratory rates. The company states this is inexpensive to produce. In a couple prior posts, I covered a bit about how the Raisin system works.
report data. The technology is called “ChipSkin”. How does this work, you swallow your pill and your pill starts talking and sending information. The information from the pill goes to a patch or a tiny device implanted under your skin and will send your heart and respiratory rates. The company states this is inexpensive to produce. In a couple prior posts, I covered a bit about how the Raisin system works.
Novartis Invests 24 Million with Proteus – Chip On A Pill Also Known as IMeds as Discussed at TEDMED
So far we are approved for the patch and device in the US and Europe gets the chip on a pill so all the way down it will be talking and sending your heart rate and other pertinent information. Compliance and data are both objectives here and again we don’t want to see data used against a patient should a dose be forgotten, etc. so privacy is a must. BD
REDWOOD CITY, Calif.--(EON: Enhanced Online News)--Proteus Biomedical Inc., a pioneer in intelligent medicine, announced today that it has received CE Mark approval to market its ingestible sensor and personal physiologic monitor system in the European Union. The CE Mark certifies that the Proteus system has met European Union consumer and health requirements. Proteus also received ISO 13485:2003 certification for the design, development and manufacture of its product system.
Proteus’s ingestible sensor and personal monitor system, called the RaisinTM System, is indicated under the CE Mark to timestamp, via ingestion, any discrete event (such as the ingestion of a specific pharmaceutical) and to record this event along with physiologic information such as heart rate, activity, body angle and patient-logged information. The unique ingestion event and personalized physiologic information are then communicated via Bluetooth to any computerized device, such as a mobile phone for emerging mHealth applications.




0 comments :
Post a Comment